Death ligand TRAIL as an alternative treatment option for malignant melanoma
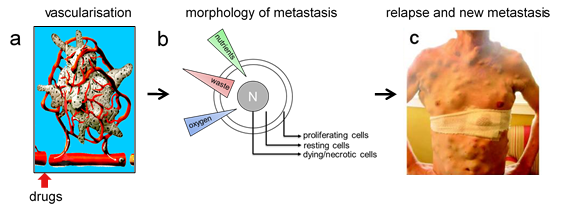
A possible explanation for the development of acquired resistance may relate to the architecture of solid tumours. The peripheral highly proliferating cell sub-population is well connected to the vascularization and it is therefore well supplied with oxygen and nutrients. With increasing distance from the vascularization, a subsequent layer of resting cells is constituted followed by a central sub-population consisting of dying cells, therefore forming the ‘necrotic’ centre, where catabolic waste accumulates.
Drug delivery is provided by the vascularisation, and may therefore predominantly affect proliferating cells in the periphery while the resting cells may receive only a minor, even sublethal drug dose, being due to drug dilution upon diffusion through the tumour tissue. Accordingly, the resting cells may be the ones surviving tumour treatment. Above this, cytokines being released by dying cells of the necrotic centre may influence the physiological response of the resting cells. In fact, they may additionally trigger pro-survival pathways or may even induce signalling pathways being responsible for reactivating or enhancing proliferation and migration of these resting cells, being causative for tumour relapse and de novo metastasis formation.
We investigate how the adaption to sublethal drug doses may affect individual tumour cell behaviour and drug susceptibility aiming to identify alternative and more effective treatment options.
To this end, alternative treatment options as well as reliable stratification tools are demanded to identify responders to selected combination therapies while sparing unnecessary treatment burden for non-responders.
Besides MAPK inhibition, individual or combined direct cell death induction with the tumour selective death ligand TRAIL (Tumour necrosis factor-Related Apoptosis-Inducing Ligand) might serve as an alternative treatment option. Novel 2nd generation TRAIL receptor-targeted agonists consisting of hexameric scTRAIL-Fc fusion proteins have been developed with improved bioactivity enhancing the cytotoxic capacity toward cancer cells.By conditioning cells to sublethal as well as lethal doses of the hexameric TRAIL receptor agonist IZI1551, we provided tools to investigate the long term therapeutic effects, aiming to gain deeper insights into mechanisms underlying drug resistance, tumour progression, and metastatic outgrowth, mimicking the condition of cells being causative of tumour relapse and secondary metastasis formation.
To uncover the underlying mechanisms of emerging TRAIL resistance and identify nodal points in the signalling network that might be used as additional therapeutic targets, a Dynamic Bayesian Network (DBN) model including apoptotic and survival pathways is applied comparing parental versus conditioned melanoma cells. Experimental data under different conditions and the prior knowledge of the network are combined, and optimal parameter values are obtained from the optimization process within the Fast Contextualization of Logical Networks (FALCON) tool, and multiple functions are enabled to analyse the different properties of the network. (Figure 2). This systems biological stratification tool may allow to identify individual changes within the tumor cell and may thereby pave the way to personalized medicine.

Relevant own publications
- Del Mistro G, Riemann S, Schindler S, Beissert S, Kontermann RE, Ginolhac A, Helder R, Presta L, Sinkkonen L, Sauter T, Kulms D: Focal adhesion kinase plays a dual role in TRAIL resistance and metastatic outgrowth of malignant melanoma. Cell Death Dis, in press (2021)
- Vetma V, Guttà C, Peters N, Praetorius C, Hutt M, Seifert O, Meier F, Kontermann R, Kulms D, Rehm M: Convergence of pathway analysis and pattern recognition predicts sensitization to latest generation TRAIL therapeutics by IAP antagonism. Cell Death Differ, doi: 10.1038/s41418-020-0512-5. (2020)
- Del Mistro G, Lucarelli P, Müller I, De Landtsheer S, Zinoveva A, Hutt M, Siegemund M, Kontermann RE, Beissert S, Sauter T, Kulms D: Systemic network analysis identifies XIAP and IκBα as potential drug targets in TRAIL resistant BRAF mutated melanoma. NPJ Syst Biol Appl 4:39; doi: 10.1038/s41540-018-0075-y (2018)
- Siegemund M, SchneiderF, Hutt M, Seifert O, Müller I, Kulms D, Pfizenmaier K, Kontermann RE: EGFR-targeted IgG-single-chain TRAIL fusion proteins for tumor therapy. Scientific Reports, doi: 10.1038/s41598-018-24450-8. (2018)
- Hutt M, Marquardt L, Seifert O, Siegemund M, Müller I, Kulms D, Pfizenmaier K, Kontermann RE: Superior properties of Fc-comprising scTRAIL fusion proteins. Mol Cancer Ther, pii: molcanther.0551.2017. doi: 10.1158/1535-7163.MCT-17-0551. (2017)
- Bullenkamp J, Raulf N, Ayaz B, Walczak H, Kulms D, Thavaraj S, Odell E, Tavassoli M: Bortezomib sensitises TRAIL-resistant HPV positive head and neck cancer cells to TRAIL through a caspase-dependent, E6-independent mechanism. Cell Death Dis,5:e1489. doi: 10.1038/cddis.2014.455. (2014)
- Raulf N, El-Attar R, Kulms D, Lecis D, Delia D, Walczak H, Papenfuss K, Odell E, Tavassoli M: Differential response of head and neck cancer cell lines to TRAIL or Smac mimetics is associated with the cellular levels and activity of caspase-8 and caspase-10. Br J Cancer, 111:1955-64. doi: 10.1038/bjc.2014.521. (2014)
- Hörnle M, Peters N, Thayaparasingham B, Vörsmann H, Kashkar H, Kulms D: Caspase-3 cleaves XIAP in a positive feedback loop to sensitize melanoma cells to TRAIL-induced apoptosis. Oncogene 30: 575-587 (2010)
- Thayaparasingham B, Kunz A, Kulms D: Sensitization of Melanoma Cells to TRAIL by UVB-induced and NFB-mediated Downregulation of xIAP. Oncogene 28: 345-362 (2009)
- Zeise E, Weichenthal M, Schwarz T, Kulms D: Resistance of human melanoma cells against the death ligand TRAIL is reversed by ultraviolet-B radiation. J Invest Dermatol 123: 746-754 (2004)
- Kulms D, Zeise E, Pöppelmann B, Schwarz T: DNA damage, death receptor activation and reactive oxygen species contribute to ultraviolet radiation-induced apoptosis in an essential and independent way. Oncogene 21: 5844-5851 (2002)
- Kulms D, Düßmann H, Pöppelmann B, Ständer S, Schwarz A, Schwarz T: Apoptosis induced by disruption of the cytoskeleton is mediated via activation of CD95 (Fas/APO-1). Cell Death Diff 9: 598-608 (2002)
- Kulms D, Pöppelmann B, Yarosh D, Luger T, Krutmann J, Schwarz T: Nuclear and cell membrane effects contribute independently to the induction of apoptosis in human cells exposed to UVB radiation. Proc Natl Acad Sci USA 96: 7974-7979 (1999)
- Kothny-Wilkes G, Kulms D, Luger TA, Kubin M, Schwarz T: Interleukin-1 protects transformed keratinocytes from tumor necrosis factor-related apoptosis-inducing ligand- and CD95-induced apoptosis but not from ultraviolet radiation-induced apoptosis. J Biol Chem 274: 28916-28921 (1999)
- Aragane Y, Kulms D, Metze D, Kothny G, Pöppelmann B, Luger TA, Schwarz T: Ultraviolet light induces apoptosis via direct activation of CD95 (FAS/APO-1) independently from its ligand CD95L. J Cell Biol 140: 171-182 (1998)
- Kothny-Wilkes G, Kulms D, Pöppelmann B, Luger TA, Kubin M, Schwarz T: Interleukin-1 protects transformed keratinocytes from TRAIL- induced apoptosis. J Biol Chem 273: 29247-29253 (1998)
- Aragane Y, Kulms D, Luger TA, Schwarz T: Downregulation of interferon -activated STAT1 by UV light. Proc Natl Acad Sci USA 94: 11490-11495 (1997)

